25/11/2024
Spinal Muscular Atrophy (SMA) is a rare autosomal recessive neurological-muscular disorder.
The cause of SMA is a homozygous or compound heterozygous mutations in the SMN1 gene, which results in insufficient production of the SMN protein necessary for nerve-muscle communication.
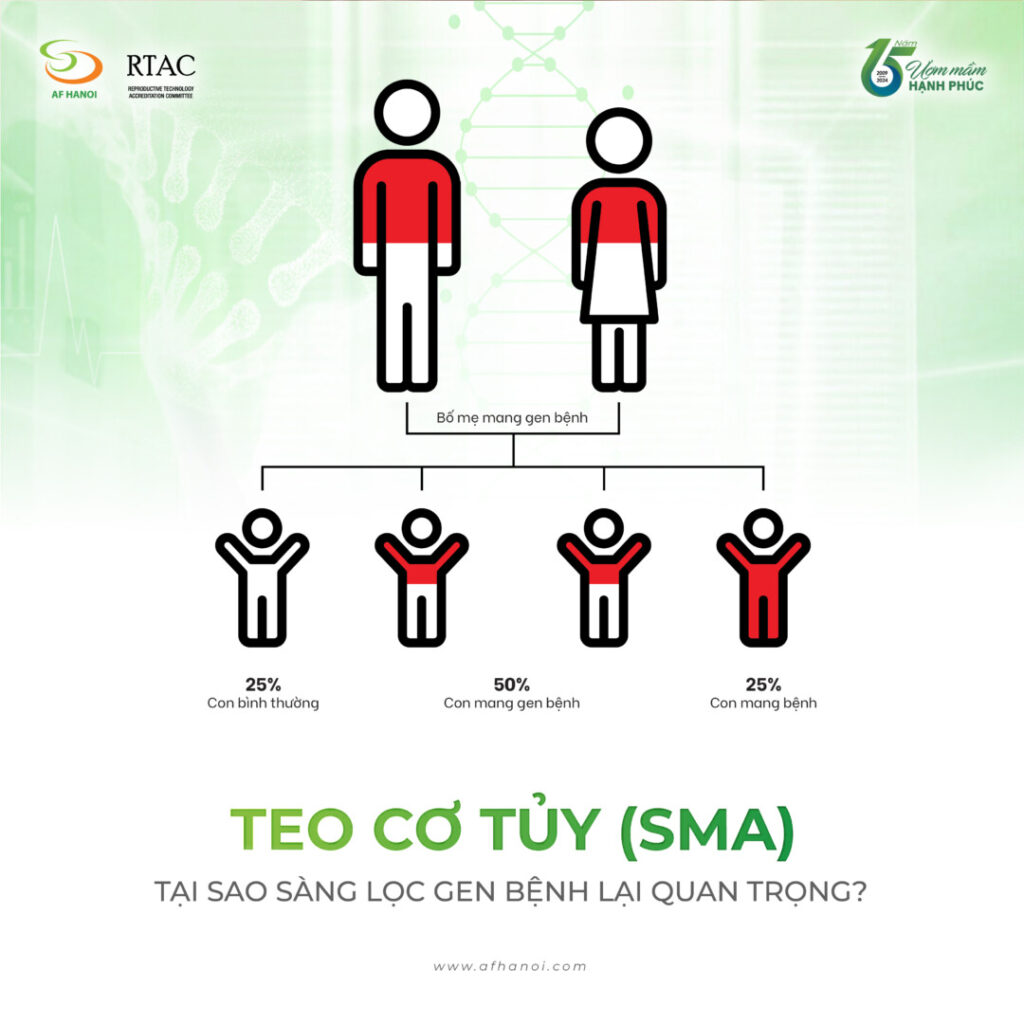
Individuals who are heterozygous carriers of one SMN1 pathogenic mutation are asymptomatic and do not show any symptoms of the disease. However, a couple with both partners being carriers may have a 25% chance of having a child affected by SMA in each pregnancy.
Prevalence of SMA and Carrier Frequency
The carrier frequency in the general population is 1 in 50. The incidence of SMA is approximately 1 in 10,000 live births, affecting both males and females across all races.
SMA Newborn Screening Methods
Newborn SMA screening can be incorporated into routine newborn screening programs to detect other disorders in the first few days of life. Newborn SMA screening helps identify infants at high risk for SMA through dried blood spot testing from the newborn’s heel, which has been collected for screening other disorders. Infants who are at risk of SMA will need further testing (genetic testing) to confirm the diagnosis and clinical follow-up to assess the type of SMA.
Carrier Screening Methods
• According to the guidelines for carrier screening from the American College of Medical Genetics (ACMG) and the American College of Obstetricians and Gynecologists (ACOG), SMA is one of the diseases that should be screened before marriage and before pregnancy.
• Genetic testing helps screen for the risk of carrying the SMN1 gene mutation in both partners.
Why is Carrier Screening and Newborn SMA Screening Important?
SMA is one of the leading causes of death in newborns due to genetic inheritance. Treatment methods have been developed and shown to be significantly effective in improving mobility (gene therapy); however, these are mainly effective for infants without severe symptoms, respiratory failure, and particularly, the cost is very high. Identifying carrier couples and providing prenatal counseling can help prevent the birth of children with SMA. Early detection and follow-up for infants identified with the disease-causing mutation allow for early intervention and treatment, improving quality of life and survival rates.
